Red-emitting dyes based on phenothiazine-modified 2-hydroxychalcone analogues: mechanofluorochromism and gelation-induced emission enhancement†
Abstract
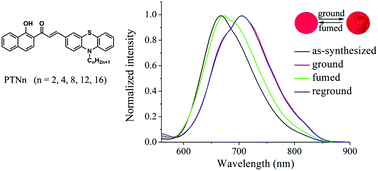
New D–π–A type phenothiazine-modified 2-hydroxychalcone analogues PTNn (n = 2, 4, 8, 12, and 16) have been synthesized, and they exhibited reversible mechanofluorochromism. Notably, the emission of PTNn in different solid-states was in the range of 600–800 nm, and red-emitting mechanofluorochromic (MFC) materials were seldom reported. The as-synthesized crystals of PTN16 emitted red light centered at 667 nm, and were changed into deep red emitting ground powders after grinding. The fluorescence emission could be recovered to the original state when the ground powders were fumed with the DCM vapor or heated. The MFC processes were accompanied by the transformation between crystalline and amorphous states, which could be confirmed based on the results of XRD patterns in different solid-states. Additionally, it was found that the length of the alkyl chain affected the performance in mechanofluorochromism for PTNn. The dye bearing a short carbon chain showed high-contrast MFC behavior compared with the one bearing a long carbon chain. Moreover, PTN12 and PTN16 bearing long carbon chains could self-assemble into organogels in a certain amount of organic solvents, and gelation-induced emission enhancement was observed during organogelation.


 Please wait while we load your content...
Please wait while we load your content...