Controlled synthesis of a Bi2O3–CuO catalyst for selective electrochemical reduction of CO2 to formate†
Abstract
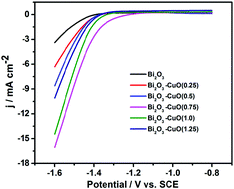
The electro-reduction of CO2 to produce energy sources has been considered as a visionary pathway with the help of renewable electricity, which can achieve carbon neutrality and mitigate global warming. Nevertheless, developing a high selectivity, good activity and superior stability catalyst is a big challenge. Here, Bi2O3–CuO(x) bimetallic oxide catalysts were synthesized by a facile coordination–precipitation method with concisely controlled atomic ratios (Cu/Bi). They exhibit a remarkable performance for sufficient reduction of CO2 to formate, achieving a maximum faradaic efficiency of 89.3% at a potential of −1.4 V vs. SCE. The catalysts are shown to be robust during 10 h of uninterrupted electrolysis. The notable catalytic activity suggests that controlling the Cu/Bi molar ratio is a key factor in developing special micro-structure Bi2O3–CuO(x) catalysts for electrochemical reduction of CO2 to formate in aqueous systems.


 Please wait while we load your content...
Please wait while we load your content...