Degradation of lignin with aqueous ammonium-based ionic liquid solutions under milder conditions†
Abstract
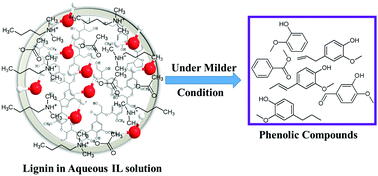
This study investigates the performance of two aqueous ionic liquids (ILs), dimethylbutylammonium acetate ([DMBA][Ac]) and dimethylbutylammonium butanoate ([DMBA][B]), solutions for depolymerizing alkali lignin into valuable phenolic compounds. The favorable operation conditions, including reaction temperature and reaction time, are explored. The extent of depolymerization of the lignin is evaluated by analysis with gel permeation chromatography (GPC). The results show that the average molecular weights of the depolymerized lignin samples can be reduced by as high as 93.8% and 86.8% after treating with the aqueous [DMBA][Ac] and [DMBA][B], respectively. Moreover, the aromatic chemical species in the depolymerized solutions are identified by using gas chromatography−mass spectrophotometry (GC-MS). The confirmation of the chemical species is further made by using a series of spectroscopic techniques, such as FT-IR, and 1H NMR and 13C NMR spectroscopy. Promising results have been achieved for the depolymerization of the lignin into valuable chemicals by using the proposed green media, aqueous solutions of ionic liquids [DMBA][Ac] and [DMBA][B], under milder conditions.


 Please wait while we load your content...
Please wait while we load your content...