Intrinsic role of pH in altering catalyst properties of NiMoP over alumino-silicate for the vapour phase hydrodeoxygenation of methyl heptanoate†
Abstract
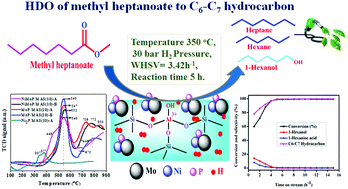
Monometallic and bimetallic Ni2P, MoP, and NiMoP active species were successfully impregnated on thermally stable, high surface area mesoporous alumino-silicate with an Si/Al ratio of 10 at room temperature via a facile wet impregnation method under both acidic and basic conditions using HCl and NH4OH as pH regulators, respectively. Furthermore, the intrinsic role of pH in altering the physicochemical properties of the catalysts was comprehensively evaluated. The catalysts were tested in a high-pressure stainless steel fixed bed reactor at different temperatures ranging from 275–350 °C, under 10–40 bar hydrogen pressure for the hydrodeoxygenation (HDO) of methyl heptanoate. The reaction pathway and product distribution of methyl heptanoate were manifested at different temperatures and pressures. The HDO activity and synergistic factor were found to be remarkably higher for the NiMoP/MAS (10)-A catalyst than the NiMoP/MAS (10)-B catalyst and its monometallic counterparts. This investigation proves that the NiMoP/MAS (10)-A catalyst is a promising catalyst for green fuel production from non-edible oils through hydrodeoxygenation. It was also unequivocally confirmed that the catalytic process does not suffer from any mass transfer resistance; thus, making the scaling up of the reaction more feasible.


 Please wait while we load your content...
Please wait while we load your content...