Pyrazoline-based colorimetric and fluorescent probe for detection of sulphite†
Abstract
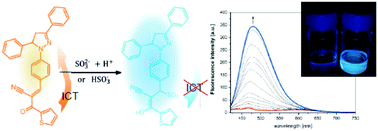
Two pyrazoline-based fluorescent and colorimetric probes have been synthesized and their photophysical properties have been investigated by means of electronic absorption and emission spectroscopy. The compounds differ from each other by the presence of a phenyl or thiophene end group attached to the α,β-unsaturated ketone. The probes detect sulphite anions in the Michael addition reaction to the α,β-unsaturated ketone with a high selectivity and good sensitivity (7.56 μM for phenyl and 4.87 μM for the thiophene counterpart). Here, the optical response is based on the recovery of triphenylpyrazoline fluorescence, which is largely blue-shifted as compared to weak charge transfer emission of the sensors. This large hypsochromic shift of the emission maximum along with a strong fluorescence enhancement (up to Isulphite/I0 = 43) can be an advantage in terms of the accurate evaluation of the fluorescence intensity ratio. The pyrazoline-based sensor decorated with thiophene is able to detect sulphite species in river water solutions with a good selectivity and sensitivity.


 Please wait while we load your content...
Please wait while we load your content...