Metallic oxide-modified sulfated zirconia: an environment-friendly solid acid catalyst
Abstract
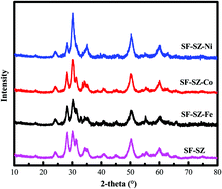
Metallic oxides were introduced into the synthesis of sulfated zirconia. Catalytic activity was tested by an olefin removal reaction. By combining the analysis from the aspect of catalyst morphology, sulfur state and surface acid property, we concluded that the incorporation of metallic oxides has three important positive effects on the environmental friendly solvent-free preparation: (i) it inhibited the phase transition during calcination and promoted the existence of tetragonal ZrO2, which is recognized as the valid phase; (ii) it increased the amount of S, especially S6+, which is the source of active sites; and (iii) it adjusted the surface acid property, extended the single service lifetime and reduced the deactivation rate.


 Please wait while we load your content...
Please wait while we load your content...