Highly active enzymes immobilized in large pore colloidal mesoporous silica nanoparticles†
Abstract
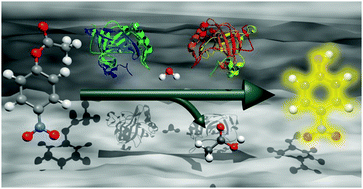
Various bio-applications of mesoporous materials (e.g., the immobilization of enzymes or the delivery of biomolecules such as siRNA) require large pores for the successful adsorption of the rather large molecules of interest and protecting the fragile cargo from external forces such as degradation. We describe the facile synthesis of functionalized mesoporous silica nanoparticles with large pores (LP-MSNs) providing high loading capacity for the immobilization of two differently-sized enzymes. The synthesis procedure yields homogeneous core–shell particles of about 100 nm in size with large mesopores (about 7 nm in diameter) and an azide-functionality inside the pores. The LP-MSNs were synthesized employing a co-condensation approach with the rather large micellar template cetyltrimethylammonium p-toluenesulfonate (CTATos). Due to the azide functionality, the LP-MSNs are suitable for bio-orthogonal click chemistry reactions within the porous network. Two different acetylene-functionalized enzymes (sp-carbonic anhydrase (CA) and sp-horseradish peroxidase (HRP)) were immobilized in the pores of the obtained LP-MSNs by a copper-catalyzed 1,3-dipolar cycloaddition reaction. The covalent attachment of the enzymes within the mesopores allowed us to investigate the catalytic performance of the enzyme–silica systems. The enzymes are stable after bioconjugation with the silica support and show high catalytic activity over several cycles for the colorimetric reaction of guaiacol (2-methoxyphenol) in case of LP-MSN–HRP and the hydrolysis of 4-nitrophenyl acetate (NPA) by LP-MSN–CA.
- This article is part of the themed collection: The Creative World of Porous Materials


 Please wait while we load your content...
Please wait while we load your content...