Design, synthesis, and preliminary biological evaluation of 3′,4′,5′-trimethoxy flavonoid salicylate derivatives as potential anti-tumor agents†
Abstract
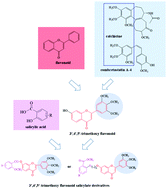
According to the pharmacophore combination principle, a set of new 3′,4′,5′-trimethoxy flavonoid salicylate derivatives were designed, synthesized, and evaluated for biological activity. The cytotoxicity evaluation revealed that compound 10v exhibited higher potency than 5-Fu against HCT-116 cells. Preliminary biological activity studies showed that compound 10v could inhibit the colony formation and migration of HCT-116 cells. Besides, the Hoechst 33258 staining assay and flow cytometry revealed that treatment with compound 10v induced the apoptosis of HCT-116 cells in a concentration-dependent manner, while it had no effect on their cell cycle. The WB analysis suggested that HIF-1α, tubulin, HK-2, and PFK might be the potential pharmacophore targets of compound 10v. Tubulin was a potential drug target for compound 10v, which was explained by analyzing the crystal structure of compound 10v complexed with tubulin. These results indicated that compound 10v might be a promising anti-tumor agent candidate, deserving further optimization and evaluation.


 Please wait while we load your content...
Please wait while we load your content...