The effects of molecular structure and functional group of a rodlike Schiff base mesogen on blue phase stabilization in a chiral system†
Abstract
Two types of racemic rodlike Schiff base mesogens with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (type I) and –N
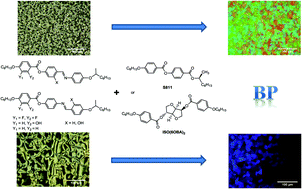
N– (type I) and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– (type III) linkages were prepared. These mesogens possessed either difluoro substitutions at the inner-core position of the phenyl ring or hydroxy group to form intramolecular hydrogen bonding with an ester or/and imine linkage. When the appropriate concentration of chiral additive is doped into them, the incorporation of two fluoro substituents is more useful for blue phase (BP) stabilization than that of a hydroxy group near the ester linkage in Schiff base mesogens. BPI and BPII can be identified by reflectance spectra and polarized optical microscope images. BPII emerges easily on cooling when the appropriate chiral dopant ISO(6OBA)2 or chiral dopant S811 is doped into the Schiff base mesogen having only a hydroxy group near the ester linkage. Interestingly, BPI can be observed when 10–15 wt% ISO(6OBA)2 was doped into the difluoro substituted Schiff base mesogen III during a heating process. The experimental and molecular modeling results indicate that most of the difluorinated Schiff base mesogens with larger dipole moments exhibit wider BP ranges than their corresponding non-fluorinated homologues under the same chirality condition. In addition, wide BPs can be induced for racemic rodlike Schiff base mesogens I in the chiral system and this is easier than that for racemic rodlike Schiff base mesogens III. In Schiff base mesogens I, the dipole moment is dominant for BP stabilization. However, the fluorine substituent effect is the main factor in Schiff base mesogens III.
C– (type III) linkages were prepared. These mesogens possessed either difluoro substitutions at the inner-core position of the phenyl ring or hydroxy group to form intramolecular hydrogen bonding with an ester or/and imine linkage. When the appropriate concentration of chiral additive is doped into them, the incorporation of two fluoro substituents is more useful for blue phase (BP) stabilization than that of a hydroxy group near the ester linkage in Schiff base mesogens. BPI and BPII can be identified by reflectance spectra and polarized optical microscope images. BPII emerges easily on cooling when the appropriate chiral dopant ISO(6OBA)2 or chiral dopant S811 is doped into the Schiff base mesogen having only a hydroxy group near the ester linkage. Interestingly, BPI can be observed when 10–15 wt% ISO(6OBA)2 was doped into the difluoro substituted Schiff base mesogen III during a heating process. The experimental and molecular modeling results indicate that most of the difluorinated Schiff base mesogens with larger dipole moments exhibit wider BP ranges than their corresponding non-fluorinated homologues under the same chirality condition. In addition, wide BPs can be induced for racemic rodlike Schiff base mesogens I in the chiral system and this is easier than that for racemic rodlike Schiff base mesogens III. In Schiff base mesogens I, the dipole moment is dominant for BP stabilization. However, the fluorine substituent effect is the main factor in Schiff base mesogens III.


 Please wait while we load your content...
Please wait while we load your content...