C2/C3 alkynylation of l-ascorbic acid by Sonogashira coupling and efficient access to some potent and highly selective novel anticancer agents†
Abstract
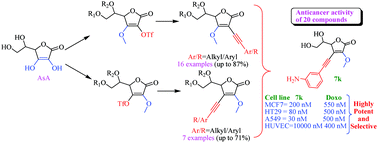
Natural products are a valuable source of bioactive compounds for the development of new drugs. Herein, we report an undemanding synthesis of novel C2/C3 alkynyl derivatives of L-ascorbic acid (AsA) via the Sonogashira cross-coupling reaction with moderate to good yield. All new derivatives exhibited superior potency against human MCF7, A549 and HT29 cancer cell lines. Compound 7k showed 2.7, 16.6 and 6.2 fold more potency against MCF7, A549 and HT29 cell lines, respectively, compared to doxorubicin and 643 fold more potency compared to AsA against the MCF7 cancer cell line. Notably, it showed 25 fold less cytotoxicity towards the normal human umbilical vein endothelial cell (HUVEC) line as compared to the doxorubicin anticancer drug. Pleasantly, it showed 333 times more cytotoxicity to human cancer cells compared to human normal cells. The flow cytometry study indicated that compound 4h and 7k arrest A549 cells in the G0/G1 phase via apoptosis. Furthermore, these compounds have high potential to be developed as promising anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...