Synthesis and electronic properties of A3B-thienyl porphyrins: experimental and computational investigations†
Abstract
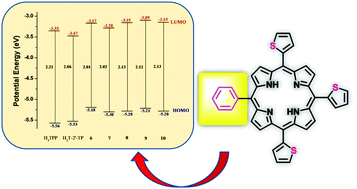
A convenient reaction protocol has been explored for the synthesis of A3B-type meso substituted porphyrins containing three thien-2′-yl groups and one subsituted phenyl group. 100–150 mg of pure A3B thienyl porphyrins was obtained from a single reaction in the case of 4-hydroxyphenyl, 3-nitrophenyl, 3,4,5-trimethoxyphenyl, 3,4-dimethoxyphenyl and 4-pyridyl substituents. NMR, UV-vis, fluorescence and electrochemical studies were conducted using pure products to understand the effect of the nature of the meso phenyl group on the electronic properties. X-ray structural analysis of thienyl porphyrins containing 4-hydroxyphenyl and 3,4,5-trimethoxyphenyl residues confirmed the substituent regiochemistry and the conformations of the thienyl groups in the solid state. The UV-vis and fluorescence bands experienced modest blue shifts when compared to meso-tetrathien-2′-yl porphyrin (H2T-2′-TP). The redox potentials showed cathodic shifts with respect to H2T-2′-TP and revealed variation of frontier orbital energy levels depending upon the phenyl substituent. Computational DFT and TD-DFT studies on all the phenyl derivatives confirmed the role of the phenyl substituent in modulating the HOMO and LUMO energy levels and agree well with the observed effects of the optical bands and the redox potentials.


 Please wait while we load your content...
Please wait while we load your content...