Green solvent assisted synthesis of cesium bismuth halide perovskite nanocrystals and the influences of slow and fast anion exchange rates†
Abstract
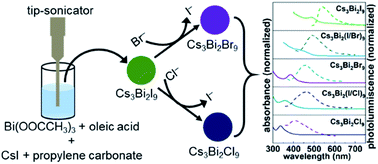
Replacing lead in halide perovskites to address the concerns of their toxicity and stability has driven a recent surge in research toward alternative lead-free perovskite materials. Lead-free all inorganic cesium bismuth halide (Cs3Bi2X9) perovskite nanocrystals have attracted attention in recent years due to the air-stability and non-toxic nature of bismuth. Herein, we demonstrate a facile sonication-assisted approach for the preparation of all-inorganic cesium bismuth iodide (Cs3Bi2I9) perovskite nanocrystals (NCs) using propylene carbonate as a green, alternative solvent. The photoluminescence (PL) spectra of the Cs3Bi2X9 NCs have a peak emission that can be tuned from 410 to 550 nm by controlling the composition of the NCs through an anion exchange reaction using tetraalkylammonium halides as a source of halide ions. The rate of this anion exchange reaction is demonstrated to have a significant influence on the dimensions of the NCs obtained from the parent Cs3Bi2I9 NCs. The PL emission of these nanocrystals is predominately due to exciton recombination processes. The NCs also exhibit air-stability for at least 150 days.
- This article is part of the themed collection: Nanoscale Advances Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...