Tailoring the stability, photocatalysis and photoluminescence properties of Au11 nanoclusters via doping engineering†
Abstract
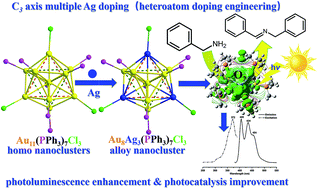
Dopants in gold nanoclusters have been proved to mediate the intrinsic electronic properties of homo-clusters. In this work, we report the precise synthesis of atomically precise Au8Ag3(PPh3)7Cl3 alloy nanoclusters with multiple Ag dopants for the first time. Their structure was resolved by single-crystal X-ray crystallography. Au8Ag3(PPh3)7Cl3 nanoclusters possessed a similar structure topology to the well-known Au11(PPh3)7Cl3 nanoclusters. It is observed that the three Ag atoms were fixed at the cluster surface and bound selectively with the chlorine ligands in a C3-axis manner. The alloy nanoclusters exhibited a closed-shell electronic structure (i.e., 8(Au 6s1) + 3(Ag 5s1) − 3(Cl) = 8e), as evidenced by electrospray ionization-mass spectrometry (ESI-MS). The photothermodynamic stability of alloy clusters was remarkably improved (e.g., full decomposition after 7 days under sunlight irradiation vs. 3 days for Au11(PPh3)7Cl3 clusters). DFT calculations indicated that the Ag dopants in a C3-axis manner could obviously delocalize the electrons of Au to the orbitals of P atoms and then mediate the electronic property of the clusters. Shrinkage of the HOMO–LUMO gap to 1.67 eV of Au8Ag3(PPh3)7Cl3 was observed as compared with that of homo-nanoclusters of Au11(PPh3)7Cl3 (2.06 eV). The electrochemical gap of Au8Ag3(PPh3)7Cl3 alloy nanoclusters was 1.272 V, which was higher than that of Au11(PPh3)7Cl3 nanoclusters, which indicated higher electrochemical stability, as evidenced by the differential pulse voltammetry (DPV) method. Au8Ag3(PPh3)7Cl3 clusters exhibited three specific photoluminescence peaks at 405, 434 and 454 nm. AuAg alloy clusters exhibited twofold greater activity than homo gold clusters in the photooxidation of benzylamine, which was mainly due to the unique electronic properties of the alloy clusters. Controllable heteroatom doping engineering is a powerful method to tune the electronic properties of clusters, and then improve their photothermodynamic and electrochemical stability simultaneously for potential photocatalytic applications.
- This article is part of the themed collections: Editor’s Choice: Single-atom and nanocluster catalysis and Photocatalysis and Photoelectrochemistry


 Please wait while we load your content...
Please wait while we load your content...