Parameters affecting the synthesis of carbon dots for quantitation of copper ions†
Abstract
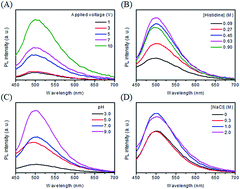
A simple, eco-friendly, and low-cost electrochemical approach has been applied to the synthesis of carbon dots (C dots) from histidine hydrochloride in the absence or presence of halides (Cl, Br, and I) at various potentials up to 10 V. The as-formed C dots refer to C dots, Cl–C, Br–C, and I–C dots. The time-evolution UV-vis absorption and photoluminescence (PL) spectra provide more detailed information about the formation of C dots. Upon increasing the reaction time from 1 to 120 min, more and more C dots are formed, leading to increased PL intensity. The halides play two important roles in determining the formation of C dots; controlling the reaction rate and surface states. When compared to chloride and bromide, iodide has a greater effect on varying surface states and inducing PL quenching through intersystem crossing. The PL intensities of the four types of C dots all decrease upon increasing Cu2+, Hg2+, and Ag+ concentrations. In the presence of 0.8 mM I−, I–C dots compared to C dots, Cl–C dots, and Br–C dots are slightly better for quantitation of Cu2+. Fourier transform infrared spectroscopy, cyclic voltammetry, electrochemical impedance spectroscopy, and X-ray photoelectron spectroscopy results of I–C dots reveal the interactions of Cu2+ with the surface ligands (imidazole and histidine). The I–C dot probe in the presence of 0.8 mM I− is selective toward Cu2+ over the tested metal ions such as Hg2+ and Ag+. The assay provides a limit of detection of 0.22 μM for Cu2+ at a signal-to-noise ratio of 3. Practicality of this probe has been validated by the analyses of tap, lake, and sea water samples, with negligible matrix effects.
- This article is part of the themed collection: Quantum and carbon dots


 Please wait while we load your content...
Please wait while we load your content...