Surfactant-assisted individualization and dispersion of boron nitride nanotubes†
Abstract
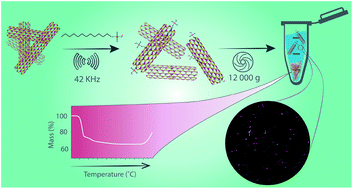
Boron nitride nanotubes (BNNTs) belong to a novel class of material with useful thermal, electronic and optical properties. However, the study and the development of applications of this material requires the formation of stable dispersions of individual BNNTs in water. Here we address the dispersion of BNNT material in water using surfactants with varying properties. The surfactants were compared based on the quantity of BNNTs dispersed and the quality of the dispersions, as visualized by AFM and cryo-TEM. All surfactants produce dispersions of individualized or small bundles of BNNTs. Of the surfactants tested, high molecular weight, nonionic surfactants suspend the most BNNTs, while ionic surfactants remove the most h-BN impurities. The surfactant dispersions were further characterized by ensemble measurements, such as UV absorption and photoluminescence, dynamic light scattering (DLS), and zeta potential to investigate dispersion stability and quality. These techniques provide a facile strategy for testing future BNNT dispersions. The results of this study reveal that BNNT dispersions in aqueous solution can be tuned to fit a specific application through surfactant selection.
- This article is part of the themed collection: Nanoscale Advances Most Popular Articles so far


 Please wait while we load your content...
Please wait while we load your content...