Photocatalytic overall water splitting on Pt nanocluster-intercalated, restacked KCa2Nb3O10 nanosheets: the promotional effect of co-existing ions†
Abstract
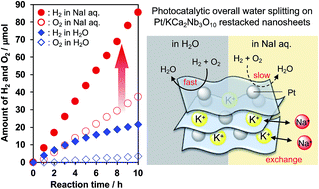
Promotional effects of co-existing ions on overall water splitting into H2 and O2 have been studied in bulk-type semiconductor photocatalysts (e.g., TiO2), but such an effect remains unexplored in two-dimensional nanosheet photocatalysts. Here we examined the effect of co-existing ions on the photocatalytic water splitting activity of Pt nanocluster-intercalated KCa2Nb3O10 nanosheets. Interestingly, not only anions, as usually observed in bulk-type photocatalysts, but also cations had a significant influence on the photocatalytic performance. The rates of H2 and O2 evolution over Pt/KCa2Nb3O10 as well as the product stoichiometry were improved in the presence of NaI. I− ions were found to effectively suppress undesirable backward reactions, consistent with the previous work by Abe et al. (Chem. Phys. Lett., 2003, 371, 360–364). On the other hand, Na+ ions in the reaction solution were exchanged for K+ in the interlayer space of KCa2Nb3O10 during the water splitting reaction, which promoted interlayer hydration and consequently improved photocatalytic performance.
- This article is part of the themed collection: Photocatalysis and Photoelectrochemistry


 Please wait while we load your content...
Please wait while we load your content...