Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage†
Abstract
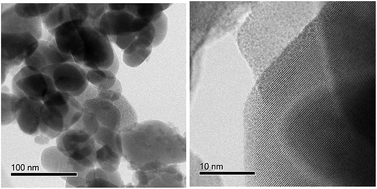
The design of a suitable electrode is an essential and fundamental research challenge in the field of electrochemical energy storage because the electronic structures and morphologies determine the surface redox reactions. Calcium molybdate (CaMoO4) was synthesized by a combustion route at 300 °C and 500 °C. We describe new findings on the behaviour of CaMoO4 and evaluate the influence of crystallinity on energy storage performance. A wide range of characterization techniques was used to obtain detailed information about the physical and morphological characteristics of CaMoO4. The characterization results enable the phase evolution as a function of the electrode synthesis temperature to be understood. The crystallinity of the materials was found to increase with increasing temperature but with no second phases observed. Molecular dynamics simulation of electronic structures correlated well with the experimental findings. These results show that to enable faster energy storage and release for a given surface area, amorphous CaMoO4 is required, while larger energy storage can be obtained by using crystalline CaMoO4. CaMoO4 has been evaluated as a cathode material in classical lithium-ion batteries recently. However, determining the surface properties in a sodium-ion system experimentally, combined with computational modelling to understand the results has not been reported. The superior electrochemical properties of crystalline CaMoO4 are attributed to its morphology providing enhanced Na+ ion diffusivity and electron transport. However, the presence of carbon in amorphous CaMoO4 resulted in excellent rate capability, suitable for supercapacitor applications.
- This article is part of the themed collection: Nanoscale Advances Most Popular Articles so far


 Please wait while we load your content...
Please wait while we load your content...