Effective chemical potential for non-equilibrium systems and its application to molecular beam epitaxy of Bi2Se3†
Abstract
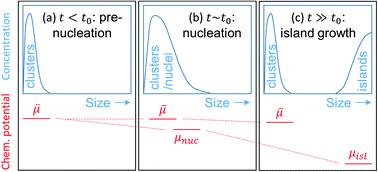
First-principles studies often rely on the assumption of equilibrium, which can be a poor approximation, e.g., for growth. Here, an effective chemical potential (![[small mu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cd.gif) ) method for non-equilibrium systems is developed. A salient feature of the theory is that it maintains the equilibrium limits as the correct limit. In application to molecular beam epitaxy, rate equations are solved for the concentrations of small clusters, which serve as feedstock for growth. We find that
) method for non-equilibrium systems is developed. A salient feature of the theory is that it maintains the equilibrium limits as the correct limit. In application to molecular beam epitaxy, rate equations are solved for the concentrations of small clusters, which serve as feedstock for growth. We find that ![[small mu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cd.gif) is determined by the most probable, rather than by the lowest-energy, cluster. In the case of Bi2Se3,
is determined by the most probable, rather than by the lowest-energy, cluster. In the case of Bi2Se3, ![[small mu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cd.gif) is found to be highly supersaturated, leading to a high nucleus concentration in agreement with experiment.
is found to be highly supersaturated, leading to a high nucleus concentration in agreement with experiment.


 Please wait while we load your content...
Please wait while we load your content...