Chemisorption of metallic radionuclides on a monolayer MoS2 nanosheet
Abstract
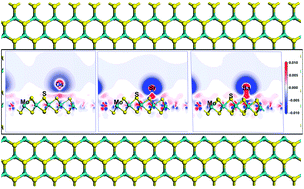
In the process of developing nuclear energy, removing radioactive waste in the environment is a challenging problem that human beings have to face. The main work of this paper is to investigate the mechanism of the interaction between metallic radionuclides (Cs, Sr, and Ba) and a monolayer MoS2 nanosheet, and the work is completed by using the first principles calculation method. The results show that all of the three kinds of metallic radionuclides can be chemisorbed on the monolayer MoS2 nanosheet. The optimum adsorption site for the metallic radionuclides adsorbed on the monolayer MoS2 is TMo (top of the Mo atom), because the metallic radionuclides can interact with the three nearest S atoms when the metallic radionuclides are at the TMo site. The chemisorption strength of the metallic radionuclides on the monolayer MoS2 is Ba > Sr > Cs. The mechanism of the chemisorption is explored by using the total charge transfer, density of states, and electron density difference. The final analysis shows that the s orbital of S atoms plays an important role in the chemisorption.
- This article is part of the themed collection: Nanoscale Advances Most Popular Articles so far


 Please wait while we load your content...
Please wait while we load your content...