Copper-binding energetics of amicyanin in different folding states†
Abstract
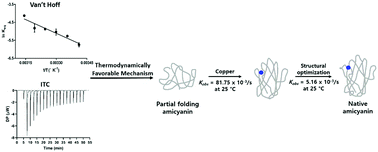
Amicyanin is a type I copper protein that mediates electron transfer between methylamine dehydrogenase and cytochrome c-551i for energy production in Paracoccus denitrificans. Although the Met98 axial ligand of amicyanin has been shown to dictate metal selectivity and specificity during protein folding, the mechanism involved in copper-mediated amicyanin folding is unknown. Here, we kinetically and spectroscopically described reaction steps for incorporating copper into fully and less folded apo-amicyanin and established thermodynamic parameters for two amicyanin folding states. The rate constant for the incorporation of copper into fully folded apo-amicyanin at 25 °C was almost 1.5-fold lower than that for the initial phase of copper addition to the less folded apo-amicyanin. However, the rate constant was 10-fold higher than that of the second phase of copper addition to less folded apo-amicyanin at 25 °C. When overall binding energetic parameters (ΔH° and ΔS°) for the incorporation of copper into fully folded apo-amicyanin were measured by the van’t Hoff method and isothermal titration calorimetry, the values were more positive than those determined for less folded apo-amicyanin. This indicates that during amicyanin biogenesis, copper rapidly binds to an unfolded apo-amicyanin active site, inducing protein folding and favorably influencing subsequent organization of copper ligands.


 Please wait while we load your content...
Please wait while we load your content...