Unique roles of iron and zinc binding to the yeast Fe–S cluster scaffold assembly protein “Isu1”†
Abstract
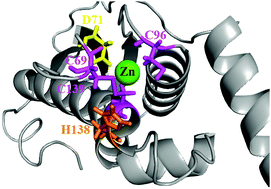
Mitochondrial Fe–S cluster biosynthesis is accomplished within yeast utilizing the biophysical attributes of the “Isu1” scaffold assembly protein. As a member of a highly homologous protein family, Isu1 has sequence conservation between orthologs and a conserved ability to assemble [2Fe–2S] clusters. Regardless of species, scaffold orthologs have been shown to exist in both “disordered” and “structured” conformations, a structural architecture that is directly related to conformations utilized during Fe–S cluster assembly. During assembly, the scaffold helps direct the delivery and utilization of Fe(II) and persulfide substrates to produce [2Fe–2S] clusters, however Zn(II) binding alters the activity of the scaffold while at the same time stabilizes the protein in its structured state. Additional studies confirm Zn binds to the scaffold's Cys rich active site, and has an impact on the protein's ability to make Fe–S clusters. Understanding the interplay between Fe(II) and Zn(II) binding to Isu1 in vitro may help clarify metal loading events that occur during Fe–S cluster assembly in vivo. Here we determine the metal : protein stoichiometry for Isu1 Zn and Fe binding to be 1 : 1 and 2 : 1, respectively. As expected, while Zn binding shifts the Isu1 to its structured state, folding is not influenced by Fe(II) binding. X-ray absorption spectroscopy (XAS) confirms Zn(II) binds to the scaffold's cysteine rich active site but Fe(II) binds at a location distinct from the active site. XAS results show Isu1 binding initially of either Fe(II) or Zn(II) does not significantly perturb the metal site structure of alternate metal. XAS confirmed that four scaffold orthologs bind iron as high-spin Fe(II) at a site composed of ca. 6 oxygen and nitrogen nearest neighbor ligands. Finally, in our report Zn binding dramatically reduces the Fe–S cluster assembly activity of Isu1 even in the presence of frataxin. Given the Fe-binding activity we report for Isu1 and its orthologs here, a possible mechanism involving Fe(II) transport to the scaffold's active site during cluster assembly has been considered.


 Please wait while we load your content...
Please wait while we load your content...