Stability of Cu(ii) complexes with FomA protein fragments containing two His residues in the peptide chain†
Abstract
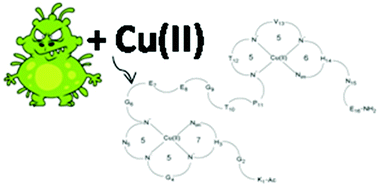
The coordination of Cu(II) ions by the Ac-KGHGNGEEGTPTVHNE-NH2 (1L) peptide – a FomA protein fragment of Fusobacterium nucleatum – and its cyclic analogue: cyclo(KGHGNGEEGTPTVHNE) (2L) was studied by potentiometric titration, spectroscopic methods (UV-Vis, CD, EPR) and mass spectrometry (MS). Both the ligands contain two histydyl residues located in the third and fourteenth position of the peptide chain. For the 1L and 2L ligands mono- and dinuclear complexes were identified and studied in an aqueous solution. At the pH range characteristic of the intestinal environment (5.5–7.5), copper(II) complexes were identified and their formation constants were determined. The same forms of the complexes with respectively the linear peptide and the cyclic peptide show similar stability, but greater than that reported in the literature for complexes with the same coordination mode. Moreover, the 1L peptide and its complex exhibit an α-helix structure, whereas the 2L peptide adopts this secondary structure only after coordination with the metal ion.


 Please wait while we load your content...
Please wait while we load your content...