Organometallic ruthenium anticancer complexes inhibit human peroxiredoxin I activity by binding to and inducing oxidation of its catalytic cysteine residue†
Abstract
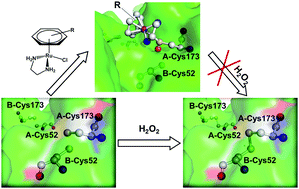
Peroxiredoxins (Prxs) are a family of ubiquitous antioxidant proteins and the inhibition of Prxs would elevate the reactive oxygen species level so as to induce cancer cell death. The interactions of organometallic ruthenium arene anticancer complexes with proteins play important roles in their mechanisms of action. Herein, we demonstrate that Ru complexes [(η6-arene)Ru(en)Cl]+ (en = ethylenediamine, arene = p-cymene (1), biphenyl (2) and 9,10-dihydrophenanthrene (3)) can inhibit the enzymatic activity of human peroxiredoxin I (Prx-I) in an order of 1 > 3 > 2. Mass spectrometric (MS) analysis revealed that 1–3 coordinated to the catalytic site Cys173 of Prx-I, and partially induced the oxidation of the thiolate to sulfenate. Quantitative MS analysis demonstrated that the higher level of the ruthenation of Cys173 is correlated with the higher inhibitory potency of the Ru complexes against Prx-I, suggesting their binding to Cys173 accounts for their inhibition towards Prx-I.
- This article is part of the themed collection: Metallomics Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...