The presence and response to Zn of ZnT family mRNAs in human dental pulp
Abstract
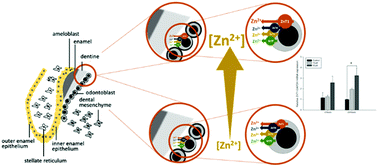
Zinc (Zn) is distributed throughout the body and within cells by saturable processes mediated by the transport proteins of the ZnT (SLC30) and ZIP (SLC39) families. The two families function in opposite directions. ZnT transporters mediate cellular zinc efflux or intracellular sequestration. Zn is found in human tooth enamel and dentine at levels that have been related to environmental exposures, such as pollution, disease, and dietary intake. The mechanism by which Zn in the odontoblast is deposited in the hard tissue of the tooth, however, is unknown but is important in determining the physical properties, and hence resilience, of enamel and in the context of the use of tooth zinc level as a biomarker of exposure. We hypothesised that zinc efflux mediated by members of the ZnT family of 10 transporters is a key step in this process and is regulated by zinc availability through effects on mRNA levels. Thus, we determined the profile of ZnT transporter mRNA in a human active-secretory odontoblast-like cell model under conditions of high- and low-extracellular Zn concentration and determined if the same transporter mRNAs were present in human dental pulp. ZnT1, ZnT5 and ZnT9 mRNAs were detected by RT-PCR in both the secretory odontoblast cells and human dental pulp. ZnT2, ZnT3 and ZnT10 mRNAs were not detected, and ZnT4 mRNA was detected in secretory odontoblasts only, which may be indicative of a specialised zinc efflux function during the active secretory phase of tooth development. ZnT1 mRNA was significantly increased in response to extracellular Zn exposure (60 μM) after 24 h. The presence of Zn transporter mRNAs in secretory odontoblasts and dental pulp indicates that the corresponding transport proteins function to deposit zinc in the dental hard tissues. The responsiveness of ZnT1 in odontoblasts to zinc availability is concordant with this being a process that is regulated to maintain cellular Zn homeostasis and that is a mediator of the relationship between environmental Zn exposure and dental Zn deposition. These findings have likely relevance to human dental health through effects of Zn transporter expression level on the hard tissue properties.


 Please wait while we load your content...
Please wait while we load your content...