Site directed mutagenesis of insulin-degrading enzyme allows singling out the molecular basis of peptidase versus E1-like activity: the role of metal ions†
Abstract
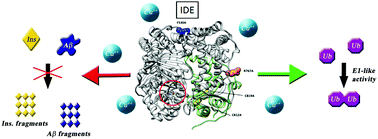
Four specifically designed IDE mutants have been used to unveil the molecular basis of peptidase versus E1-like activity of the enzyme. We have found that physiological concentrations of copper(II) ions inhibit the proteolytic activity of the enzyme towards small and large substrates but have no effect on the E1-like activity of the enzyme.


 Please wait while we load your content...
Please wait while we load your content...