In vivo metal selectivity of metal-dependent biosynthesis of cobalt-type nitrile hydratase in Rhodococcus bacteria: a new look at the nitrile hydratase maturation mechanism?†
Abstract
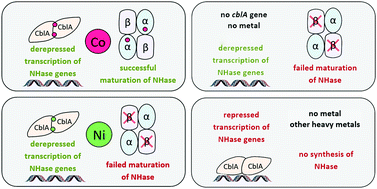
This study highlights the effect of heavy metal ions on the expression of cobalt-containing nitrile hydratase (NHase) in Rhodococcus strains, which over-produce this enzyme. Both metal-dependent derepression of transcription and maturation of NHase were considered. We demonstrated that nickel ions can derepress the NHase promoter in several Rhodococcus strains. The cblA gene of a cobalt-dependent transcriptional repressor was shown to be indispensable for nickel-mediated derepression. As for maturation, we showed that nickel ions could not replace cobalt ions during the synthesis of active NHase. We also revealed that the amount of β-subunit decreased during NHase expression without added cobalt. We showed this using three variants of NHase in vivo synthesis: by using nickel- or urea-induced synthesis in cblA+ strains, and by using metal-independent constitutive synthesis in cblA− strains. In all cases, we found that the amount of β-subunit was significantly lower than the amount of α-subunit. In contrast, equimolar amounts of both subunits were synthesized after growth in the presence of added cobalt. Nickel did not affect NHase synthesis in mixtures with cobalt. This suggests that the metal selectivity in cblA-dependent regulation of NHase transcription was too low to discriminate between cobalt and nickel, but the selectivity of the NHase maturation mechanism was high enough to do so. Moreover, we can assume that the β-subunit is more subject to proteolytic degradation without the addition of cobalt, than the α-subunit. This indicates that cobalt ions presumably play an unknown role in the stability of the β-subunit in vivo.


 Please wait while we load your content...
Please wait while we load your content...