High affinity rigidified AT2 receptor ligands with indane scaffolds†
Abstract
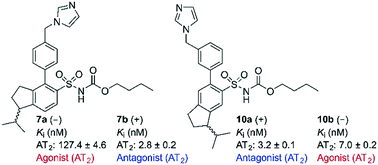
Rigidification of the isobutyl side chain of drug-like AT2 receptor agonists and antagonists that are structurally related to the first reported selective AT2 receptor agonist 1 (C21) delivered bioactive indane derivatives. Four enantiomer pairs were synthesized and the enantiomers were isolated in an optical purity >99%. The enantiomers 7a, 7b, 8a, 8b, 9a, 9b, 10a and 10b bind to the AT2 receptor with moderate (Ki = 54–223 nM) to high affinity (Ki = 2.2–7.0 nM). The enantiomer with positive optical rotation (+) exhibited the highest affinity at the receptor. The indane derivatives 7b and 10a are among the most potent AT2 receptor antagonists reported so far. As illustrated by the enantiomer pairs 7a/b and 10a/b, an alteration at the stereogenic center has a pronounced impact on the activation process of the AT2 receptor, and can convert agonists to antagonists and vice versa.


 Please wait while we load your content...
Please wait while we load your content...