Development of hydroxamate-based histone deacetylase inhibitors of bis-substituted aromatic amides with antitumor activities†
Abstract
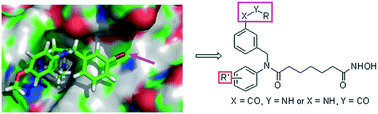
Previously, we designed and synthesized a series of bis-substituted aromatic amide-based histone deacetylase (HDAC) inhibitors. In this study, we report the replacement of a bromine atom by different amides on the phenyl ring of the CAP region. Representative compounds 9d and 10k exhibited low nanomolar IC50 values against HDAC1, which were ten times lower than that of the positive control SAHA. The IC50 of 9d against the human A549 cancer cell line was 2.13 μM. Furthermore, 9d increased the acetylation of histones H3 and H4 in a dose-dependent manner. Moreover, 9d significantly arrested A549 cells at the G2/M phase and induced A549 cell apoptosis. Finally, molecular docking investigation rationalized the high potency of compound 9d.


 Please wait while we load your content...
Please wait while we load your content...