Synthesis and structure–activity relationship studies of IgG-binding peptides focused on the C-terminal histidine residue†
Abstract
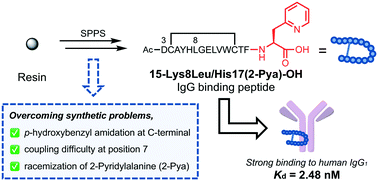
Currently, IgG-binding peptides are widely utilized as a research tool, as molecules that guide substrates to the Fc site for site-selective antibody modification, leading to preparation of a homogeneous antibody–drug conjugate. In this study, a structure–activity relationship study of an IgG-binding peptide, 15-IgBP, that is focused on its C-terminal His residue was performed in an attempt to create more potent peptides. A peptide with a substitution of His17 by 2-pyridylalanine (2-Pya) showed a good binding affinity (15-His17(2-Pya), Kd = 75.7 nM). In combination with a previous result, we obtained 15-Lys8Leu/His17(2-Pya)-OH that showed a potent binding affinity (Kd = 2.48 nM) and avoided three synthetic problems concerning the p-hydroxybenzyl amidation at the C-terminus, the difficulty associated with coupling at the His7 position and the racemization of 2-Pya.


 Please wait while we load your content...
Please wait while we load your content...