Abstract
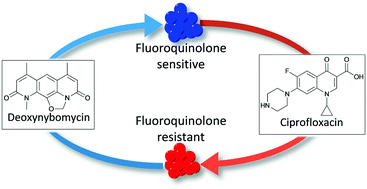
Antimicrobial resistance (AMR) is a serious issue that could have severe consequences if steps are not taken. The nybomycin natural products have the potential to extend the clinical efficacy of the marketed fluoroquinolone class of antibiotics through a ‘reverse antibiotic’ approach. However, only very limited structure–activity relationships are known for these fascinating compounds, in part due to challenges with their synthesis. Here we report a new scalable and robust synthetic route to the nybomycin natural products to aid in the development of this series. Through this synthesis, we report the antibiotic activity of novel analogues of this family confirming the selectivity for fluoroquinolone resistant bacteria and potential future opportunities for further optimisation.
- This article is part of the themed collections: Celebrating our 2021 Prizewinners and Antimicrobial Resistance


 Please wait while we load your content...
Please wait while we load your content...