Dihydropyrimidine-2-thiones as Eg5 inhibitors and L-type calcium channel blockers: potential antitumour dual agents†
Abstract
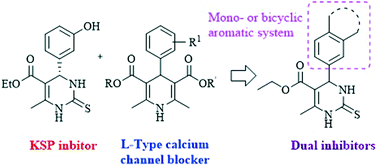
The use of multitarget drugs has evolved as an alternative to “magic bullets” for the treatment of complex diseases such as cancer, in order to affect simultaneously several targets relevant to the disease. We have designed and synthesized a series of dual agents as both Eg5 inhibitors and calcium channel blockers, bearing a 4-aryldihydropyrimidine core. Compound 2 (aryl: 3-nitrophenyl) was selected as potential dual agent due to displaying both activities: it is a vasorelaxant agent (>90% relaxation at 10−5 M in KCl-precontracted aorta rings), it decreases the response to calcium and it is cytotoxic to MCF-7 (breast), HCT-116 (colon) and A-549 (lung) cancer cell lines. The dual mechanism of action was confirmed by blocking (−)-BAY K8644-induced vascular contraction and production of monopolar spindles, typical of Eg5 inhibition. Docking suggests that both (R) and (S)-enantiomers could bind Eg5.


 Please wait while we load your content...
Please wait while we load your content...