Synthesis and discovery of asiatic acid based 1,2,3-triazole derivatives as antitumor agents blocking NF-κB activation and cell migration†
Abstract
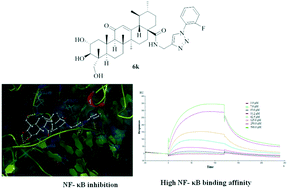
A series of asiatic acid (AA) based 1,2,3-triazole derivatives were designed, synthesized and subjected to a cell-based NF-κB inhibition screening assay. Among the tested compounds, compound 6k displayed impressive NF-κB inhibitory activity with an IC50 value in the low micromolar range. A molecular docking study was performed to reveal key interactions between 6k and NF-κB in which the 1,2,3-triazole moiety and the hydroxyl groups of the AA skeleton were important for improving the inhibitory activity. Subsequently, surface plasmon resonance analysis validated the high affinity between compound 6k and NF-κB protein with an equilibrium dissociation constant (KD) value of 0.36 μM. Further studies showed that compound 6k observably inhibited the NF-κB DNA binding, nuclear translocation and IκBα phosphorylation. Moreover, in vitro antitumor activity screening showed that compound 6k (IC50 = 2.67 ± 0.06 μM) exhibited the best anticancer activity against A549 cells, at least partly, by inhibition of the activity of NF-κB. Additionally, the treatment of A549 cells with compound 6k resulted in apoptosis induction potency and in vitro cell migration inhibition. Thus, we conclude that AA based 1,2,3-triazole derivatives may be potential NF-κB inhibitors with the ability to induce apoptosis and suppress cell migration.


 Please wait while we load your content...
Please wait while we load your content...