Neuropilin-1 peptide-like ligands with proline mimetics, tested using the improved chemiluminescence affinity detection method†
Abstract
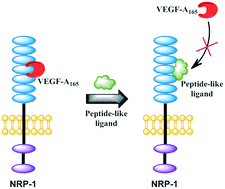
Many reports have suggested that NRP-1 acts as a co-receptor for VEGF-A165 and boosts tumour growth and metastasis. This NRP-1, due to its important role in tumour progression, triggered interest in the design of new molecules able to significantly inhibit NRP-1/VEGF-A165 interaction to suppress pathological angiogenesis. Our previous SAR studies of compounds, showing affinity for NRP-1, led us to develop branched peptides with general formula Lys(hArg)–AA2–AA3–Arg. Here, three series of analogues were synthesized, in which the middle fragment (AA2 and/or AA3) of initial sequences was substituted with unnatural Pro analogues with different rigidities and ring sizes. The synthesized compounds were screened for VEGF-A165 inhibitory activity on an improved assay (ELISA), which was selected based on our comparative inhibition study of the parent compounds, indicating that the method with chemiluminescence detection gives more accurate data. The results of affinity for NRP-1 and enzymatic stability of newly obtained compounds enabled the selection of new structures, showing a 2 and 4-fold lower IC50 value compared to parent peptides.


 Please wait while we load your content...
Please wait while we load your content...