Design, synthesis and biological evaluation of tryptamine salicylic acid derivatives as potential antitumor agents†
Abstract
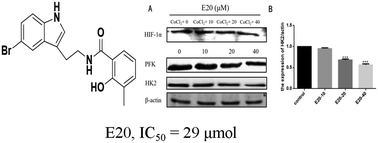
A series of tryptamine salicylic acid derivatives were synthesized and their antiproliferative activity against MGC-803, MCF-7, HepG2, A549 and HeLa cell lines was evaluated. The structure–activity relationship (SAR) study revealed that different substitutions of the C5 and C3′–C5′ positions have certain effects on the anti-proliferation activity. The growth assay revealed that N-[2-(5-bromo-1H-indol-3-yl)-ethyl]-2-hydroxy-3-methyl-benzamide (E20) showed the most potent and broad-spectrum anticancer inhibition of all the cell lines evaluated, and was only more potent than 5-Fu for the gastric cancer cell line. Preliminary studies indicated that compound E20 could inhibit colony formation and migration of MGC-803 cells. The flow cytometry (FCM) results showed that compound E20 arrested the cell cycle in the G2/M phase and induced apoptosis of MGC-803 cells in a concentration-dependent manner. In addition, the western blot results showed that E20 can down-regulate the expression of hexokinase 2. Our studies suggest that the framework of N-[2-(5-bromo-1H-indol-3-yl)-ethyl]-2-hydroxy-3-methyl-benzamide may be consider as a new type of chemical for designing effective anti-cancer drugs targeting gastric cancer cells.


 Please wait while we load your content...
Please wait while we load your content...