Quinolone-isoniazid hybrids: synthesis and preliminary in vitro cytotoxicity and anti-tuberculosis evaluation†
Abstract
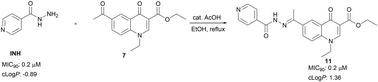
Herein, we propose novel quinolones incorporating an INH moiety as potential drug templates against TB. The quinolone-based compounds bearing an INH moiety attached via a hydrazide–hydrazone bond were synthesised and evaluated against Mycobacterium tuberculosis H37Rv (MTB). The compounds were also evaluated for cytotoxicity against HeLa cell lines. These compounds showed significant activity (MIC90) against MTB in the range of 0.2–8 μM without any cytotoxic effects. Compounds 10 (MIC90; 0.9 μM), 11 (MIC90; 0.2 μM), 12 (MIC90; 0.8 μM) and compound 15 (MIC90; 0.8 μM), the most active compounds in this series, demonstrate activities on par with INH and superior to those reported for the fluoroquinolones. The SAR analysis suggests that the nature of substituents at positions −1 and −3 of the quinolone nucleus influences anti-MTB activity. Aqueous solubility evaluation and in vitro metabolic stability of compound 12 highlights favourable drug-like properties for this compound class.


 Please wait while we load your content...
Please wait while we load your content...