Synthesis and biological evaluation against Leishmania donovani of novel hybrid molecules containing indazole-based 2-pyrone scaffolds†
Abstract
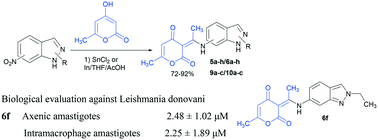
A series of novel indazole–pyrone hybrids were synthesized by a one pot reaction between N-alkyl-6(5)-nitroindazoles and 2-pyrone (4-hydroxy-6-methyl-2H-pyran-2-one) using indium or stannous chloride as the reducing system in the presence of acetic acid in tetrahydrofuran. The hybrid molecules were obtained in good to excellent yields (72–92%) and characterized by NMR and single crystal X-ray diffraction. Nineteen compounds were tested in vitro against both Leishmania donovani (MHOM/ET/67/HU3, also called LV9) axenic and intramacrophage amastigotes. Among all, five compounds showed anti-leishmanial activity against intracellular L. donovani with an IC50 in the range of 2.25 to 62.56 μM. 3-(1-(3-Chloro-2-ethyl-2H-indazol-6-ylamino)ethylidene)-6-methyl-3H-pyran-2,4-dione 6f was found to be the most active compound for axenic amastigotes and intramacrophage amastigotes of L. donovani with IC50 values of 2.48 ± 1.02 μM and 2.25 ± 1.89 μM, respectively. However, the cytotoxicity of the most promising compound justifies further pharmacomodulations.


 Please wait while we load your content...
Please wait while we load your content...