Crystallographic and SAR analyses reveal the high requirements needed to selectively and potently inhibit SIRT2 deacetylase and decanoylase†
Abstract
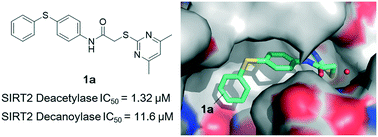
A high-quality X-ray crystal structure reveals the mechanism of compound 1a inhibiting SIRT2 deacetylase and decanoylase. Structure–activity relationship (SAR) analysis of the synthesized derivatives of 1a reveals the high requirements needed for selective inhibitors to bind with the induced hydrophobic pocket and potently inhibit sirtuin 2 deacetylase.


 Please wait while we load your content...
Please wait while we load your content...