Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs†
Abstract
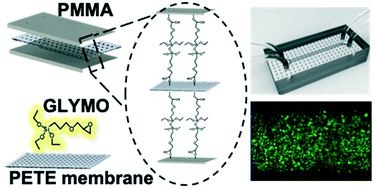
Here, we report a simple yet reliable method for bonding poly(methyl methacrylate) (PMMA) to polyethylene terephthalate (PETE) track-etched membranes using (3-glycidyloxypropyl)trimethoxysilane (GLYMO), which enables reliable cytotoxicity tests in a microfluidic device impermeable to small molecules, such as anti-cancer drugs. The porous PETE membranes treated with 5% GLYMO were assembled with microfluidic channel-engraved PMMA substrates after air plasma treatment for 1 minute, followed by heating at 100 °C for 2 minutes, which permits irreversible and complete bonding to be achieved within 1 h. The bonding strength between the two substrates (1.97 × 107 kg m−2) was robust enough to flow culture medium through the device without leakage even at a gauge pressure of above 135 kPa. For validation of its utility in drugs testing, we successfully demonstrated that human lung adenocarcinoma cells cultured in the PMMA devices show more reliable cytotoxicity results for vincristine in comparison to conventional polydimethylsiloxane (PDMS) devices due to the inherent property of PMMA of it being impervious to small molecules. Given that the current organ-on-a-chip fabrication methods mostly rely on PDMS, this bonding strategy will expand simple fabrication capability using various thermoplastics and porous track-etched membranes, and allow us to create 3D-micro-constructs that more precisely mimic organ-level physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...