Liquid biopsy using the nanotube-CTC-chip: capture of invasive CTCs with high purity using preferential adherence in breast cancer patients†
Abstract
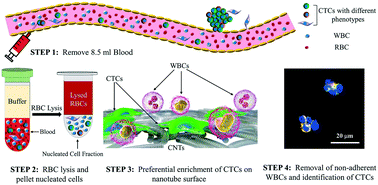
In this paper, we report the development of the nanotube-CTC-chip for isolation of tumor-derived epithelial cells (circulating tumor cells, CTCs) from peripheral blood, with high purity, by exploiting the physical mechanisms of preferential adherence of CTCs on a nanotube surface. The nanotube-CTC-chip is a new 76-element microarray technology that combines carbon nanotube surfaces with microarray batch manufacturing techniques for the capture and isolation of tumor-derived epithelial cells. Using a combination of red blood cell (RBC) lysis and preferential adherence, we demonstrate the capture and enrichment of CTCs with a 5-log reduction of contaminating WBCs. EpCAM negative MDA-MB-231/luciferase-2A-green fluorescent protein (GFP) cells were spiked in the blood of wild mice and enriched using an RBC lysis protocol. The enriched samples were then processed using the nanotube-CTC-chip for preferential CTC adherence on the nanosurface and counting the GFP cells yielded anywhere from 89% to 100% capture from the droplets. Electron microscopy (EM) studies showed focal adhesion with filaments from the cell body to the nanotube surface. We compared the nanotube preferential adherence to collagen adhesion matrix (CAM) scaffolding, reported as a viable strategy for CTC capture in patients. The CAM scaffolding on the device surface yielded 50% adherence with 100% tracking of cancer cells (adhered vs. non-adhered) versus carbon nanotubes with >90% adherence and 100% tracking for the same protocol. The nanotube-CTC-chip successfully captured CTCs in the peripheral blood of breast cancer patients (stage 1–4) with a range of 4–238 CTCs per 8.5 ml blood or 0.5–28 CTCs per ml. CTCs (based on CK8/18, Her2, EGFR) were successfully identified in 7/7 breast cancer patients, and no CTCs were captured in healthy controls (n = 2). CTC enumeration based on multiple markers using the nanotube-CTC-chip enables dynamic views of metastatic progression and could potentially have predictive capabilities for diagnosis and treatment response.


 Please wait while we load your content...
Please wait while we load your content...