Profiling protein–protein interactions of single cancer cells with in situ lysis and co-immunoprecipitation†
Abstract
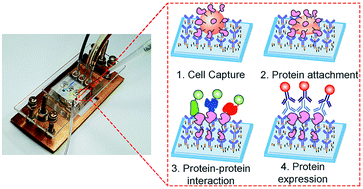
Heterogeneity in a tumor allows a small portion of cancer cells to survive and regrow upon targeted cancer therapy, eventually leading to cancer relapse. Such drug-resistant cells often exhibit dynamic adaptation of their signaling pathways at the level of protein–protein interactions (PPIs). To probe the rewiring of signaling pathways and the heterogeneity across individual cancer cells, we developed a single-cell version of the co-immunoprecipitation (co-IP) analysis that examines the amount and PPIs of target proteins immunoprecipitated from individual cells. The method captures cancer cells at predefined locations using a microfluidic chip, pulls down target proteins on the surface using antibodies, and lyses the captured cells in situ. Then, subsequent addition of eGFP-labeled downstream proteins enables the determination of the corresponding PPIs for the minimal amount of target proteins sampled from a single cell. We applied the technique to probe epidermal growth factor receptors (EGFRs) in PC9 lung adenocarcinoma cells. The results reveal that the strength of EGFR PPIs can be largely uncorrelated with the expression level of EGFRs in single cells. In addition, the individual PC9 cells showed markedly different patterns of PPIs, indicating a high heterogeneity in EGFR signaling within a genetically homogeneous population.
- This article is part of the themed collection: Single Cell Analysis


 Please wait while we load your content...
Please wait while we load your content...