A microfluidic approach for probing hydrodynamic effects in barite scale formation†
Abstract
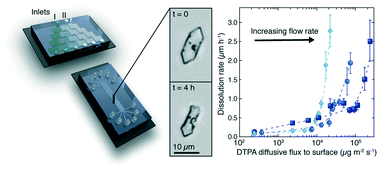
Crystallization of mineral scale components ubiquitously plagues industrial systems for water treatment, energy production, and manufacturing. Chemical scale inhibitors and/or dissolvers are often employed to control scale formation, but their efficacy in flow conditions remains incompletely understood. We present a microfluidic platform to elucidate the time-resolved processes controlling crystallization and dissolution of barite, a highly insoluble and chemically resistant component of inorganic scale, in the presence of flow. In a growth environment, increasing the flow rate leads to a crossover from a transport-limited to a reaction-limited kinetic regime. In situ optical microscopy reveals that addition of diethylenetriaminepentaacetic acid (DTPA), a common dissolution agent, alters the morphology of barite crystals grown under flow. In a dissolution environment (i.e. alkaline solutions without barium sulfate), increasing the flux of DTPA, whether by increasing the flow rate or DTPA concentration, enhances the rate of dissolution of barite. Trends in the rate of barite dissolution with DTPA concentration and flow rate indicate an optimal combination of these parameters. The combined use of microfluidics and optical microscopy provides a robust and broadly-useful platform for capturing crystallization kinetics and morphological transformation under dynamic flow conditions.


 Please wait while we load your content...
Please wait while we load your content...