3D-printing enabled micro-assembly of a microfluidic electroporation system for 3D tissue engineering†
Abstract
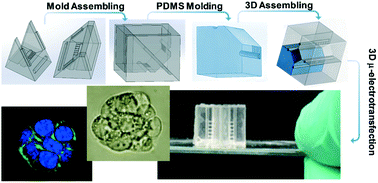
Electro-transfection is an essential workhorse tool for regulating cellular responses and engineering cellular materials in tissue engineering. However, most of the existing approaches are only focused on cell suspensions in vitro, which fails to mimic an in vivo tissue microenvironment regarding the 3D electric field distribution and mass transport in a biological matrix. However, building a 3D electro-transfection system that is compatible with 3D cell culture for mimicking the in vivo tissue microenvironment is challenging, due to the substantial difficulties in control of the 3D electric field distribution as well as the cellular growth. To address such challenges, we introduce a novel 3D micro-assembly strategy assisted by 3D printing, which enables the molding of 3D microstructures as LEGO® parts from 3D-printed molds. The molded PDMS LEGO® bricks are then assembled into a 3D-cell culture chamber interconnected with vertical and horizontal perfusion microchannels as a 3D channel network. Such a 3D perfusion microchannel network is unattainable by direct 3D printing or other microfabrication approaches, which can facilitate the highly-efficient exchange of nutrition and waste for 3D cell growth. Four flat electrodes are mounted into the 3D culture chamber via a 3D-printed holder and controlled by a programmable power sequencer for multi-directional electric frequency scanning (3D μ-electro-transfection). This multi-directional scanning not only can create transient pores all over the cell membrane, but also can generate local oscillation for enhancing mass transport and improving cell transfection efficiency. As a proof-of-concept, we electro-delivered the pAcGFP1-C1 vector to 3D cultured HeLa cells within peptide hydrogel scaffolding. The expressed GFP level from transfected HeLa cells reflects the transfection efficiency. We found two key parameters including electric field strength and plasmid concentration playing more important roles than the pulse duration and duty cycles. The results showed an effective transfection efficiency of ∼15% with ∼85% cell viability, which is 3-fold higher compared to that of the conventional benchtop 3D cell electro-transfection. This 3D μ-electrotransfection system was further used for genetically editing 3D-cultured Hek-293 cells via direct delivery of CRISPR/Cas9 plasmid which showed successful transfection with GFP expressed in the cytoplasm as the reporter. The 3D-printing enabled micro-assembly allows facile creation of a novel 3D culture system for electro-transfection, which can be employed for versatile gene delivery and cellular engineering, as well as building in vivo like tissue models for fundamentally studying cellular regulation mechanisms at the molecular level.
- This article is part of the themed collection: Lab on a Chip Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...