In situ XRF analysis of Cs adsorption by the precipitation bands of Prussian blue analogues formed in agarose gels
Abstract
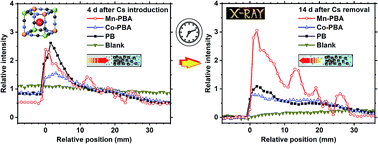
The measurement of Cs adsorption by the precipitation bands of Mn-based Prussian blue analogues (PBAs), Co-based PBAs, and Prussian blue (PB), which were spontaneously formed in agarose gel, was carried out using in situ X-ray fluorescence (XRF) spectroscopy. Three gel samples, namely “Mn-PBA,” “Co-PBA,” and “PB,” were prepared by bringing 0.05 M [Fe(CN)6]3− agarose gel (2.3 mass%) into contact with 0.50 M solutions of MnSO4, CoCl2, and FeCl2, respectively. Mn-PBA formed relatively wide, discrete precipitation bands, whereas Co-PBA and PB each formed a continuous band. The XRF intensity distribution measurements determined using an energy-dispersive XRF setup showed that the PBA/PB concentration fluctuates considerably in Mn-PBA but not in Co-PBA or PB. Time-dependent Cs L-emission distributions for these samples, into which a 0.10 M CsCl solution was introduced, indicated that in agarose gel, the precipitates of Mn-based PBAs can trap Cs+ more effectively than those of PB and Co-based PBAs if the Cs processing time is relatively long (i.e., ≥10 d). These results demonstrate the potential applicability of Mn-based PBA precipitates that are self-organized in agarose gel as pulsatile 137Cs adsorbent delivery materials.


 Please wait while we load your content...
Please wait while we load your content...