Accurate quantification of carboplatin adducts with serum proteins by monolithic chromatography coupled to ICPMS with isotope dilution analysis†
Abstract
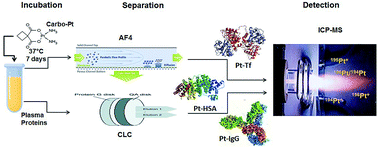
This work demonstrates for the first time the potential of monolithic chromatography coupled to double species-specific isotope dilution analysis (IDA) inductively coupled plasma mass spectrometry (ICPMS) for the detection and accurate quantification of adducts of plasma/serum proteins with carboplatin, an anti-cancer drug. The results of Pt fractionation in blood incubated with Pt drugs, to mimic cancer scenarios in real life, suggested that approximately 83% of the Pt in drug-incubated blood is found to be associated with plasma/serum proteins. Such studies were performed with cisplatin (instead of carboplatin), as cisplatin shows faster kinetics compared to carboplatin and Pt–DNA adducts have been demonstrated to be the same using both drugs. These results provide for the first time solid evidence to support that adducts of Pt drugs with plasma proteins are key competitors for the drugs binding to DNA in cancer models. The distribution of Pt associated with albumin (HSA), transferrin (Tf) and immunoglobulin (IgG) in human serum was investigated in reconstituted serum samples incubated with clinical concentrations of carboplatin for ten days at 37 °C. Two approaches were examined for the separation and detection of the three Pt–protein adducts. Asymmetrical flow field-flow fractionation (AF4) could not achieve baseline separation of the three proteins in a real matrix; however, its coupling with ICPMS was found to be particularly useful to provide detailed information on the formation of different adduct isoforms during the optimisation of the conditions for calibrant and spike production (e.g., by incubation of the individual proteins with either natural or 194Pt-enriched carboplatin). Conjoint Liquid Chromatography (CLC) based on one affinity (Protein G) and two weak anion exchange (DEAE) disks coupled to ultraviolet-visible spectroscopy (UV-Vis) and ICPMS revealed that Pt–HSA adducts represented more than 96% of the protein-bound Pt in the serum, whereas Pt–Tf and Pt–IgG represented only about 2% each. Therefore, a reference methodology based on the use of double species-specific IDA ICPMS has been developed and validated for the determination of carboplatin–HSA adducts using a simplified separation method with two DEAE monolithic disks. Limits of detection achieved for carboplatin–HSA adducts with this method were 0.005 and 0.003 ng g−1 for 194Pt and 195Pt, respectively. These values are about two orders of magnitude lower than those previously reported in the literature. An expanded uncertainty (k = 2) of 25 ng g−1 (RSU of 4.7%) was achieved for a mass fraction of carboplatin–HSA adducts of about 528 ng g−1. The reference methodology developed here will be invaluable for evaluation of dose-dependent response to treatment for individual patients in cancer trials, aimed at finding more effective therapies.
- This article is part of the themed collection: Community Leaders: Alfredo Sanz-Medel


 Please wait while we load your content...
Please wait while we load your content...