Multi-component solvent-free cascade reaction of 2-cyanoacetamides: regioselective synthesis of pyridin-2-ones bearing quaternary centers†
Abstract
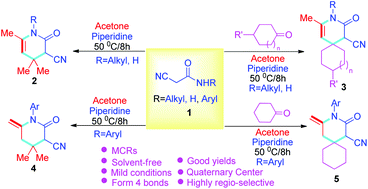
A novel protocol has been constructed for the synthesis of four types of pyridin-2-ones via solvent-free cascade reactions of 2-cyanoacetamides, various ketones, and acetone via multicomponent reactions to prepare target compounds with good to excellent yields. As a result, functionalized pyridin-2-ones bearing quaternary centers, specifically, spiropyridin-2-ones, were generated by piperidine under solvent-free conditions. Also, a double bond has been introduced into the target compounds through aryl substituted 2-cyanoacetamides under the same conditions. The advantages of this approach include no solvents, simple and practical operation (multi-component one-pot), high yields (up to 95%), and a product with potential biological activity.


 Please wait while we load your content...
Please wait while we load your content...