Visible light-promoted copper catalyzed regioselective acetamidation of terminal alkynes by arylamines†
Abstract
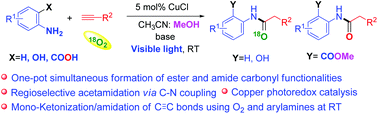
Herein, we describe a copper photoredox catalyzed synthesis of acetamide via regioselective C–N coupling of arylamines with terminal alkynes using molecular oxygen (O2) as an oxidant at room temperature under visible light irradiation (47 examples). Unique simultaneous formation of both amide and ester functionalities occurs via intramolecular cyclization in a single-step reaction in the case of anthranilic acids using inexpensive copper as a catalyst and eco-friendly O2 as an oxidant and reagent. Different substrates undergo different reaction pathways to generate similar acetamide products, as evidenced by 18O2 labelling experiments. The current protocol was also applied for the rapid, few step preparation of biologically active inhibitors (BACE-1 and PDE4). This process can be readily scaled up to a gram scale, and calculations of green metrics suggest the economic feasibility and eco-friendly nature of the current photoredox approach.


 Please wait while we load your content...
Please wait while we load your content...