Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS)†
Abstract
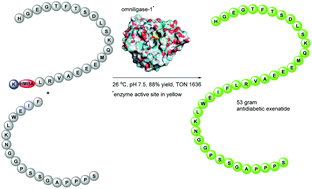
Using a large scale biocatalytic manufacture of the antidiabetic exenatide as an example, we report that chemo-enzymatic peptide synthesis (CEPS) constitutes an efficient method for sustainable manufacturing of therapeutic peptides. The H-22-39-NH2 and H-1-21-O-Cam-L-NH2 exenatide fragments requisite for the enzymatic ligations were synthesized by solid-phase peptide synthesis (SPPS) methods and in ligations of these fragments catalyzed by omniligase-1, a broad specificity ligase engineered from subtilisin BPN’, we found that at pH 9.1/37 °C a substantial formation of deamidation impurities occurs. Consequently, a more robust ligation protocol (pH 7.5, rt, 10% MeCN as co-solvent) was developed and used in efficient scale-up ligations of the two fragments both as crude peptides as well as in their purified forms. Further, the aromatic 4-hydroxymethylbenzoic acid (HMBA) was evaluated as a more robust alternative for the carboxamidomethyl (O-Cam linker), which exhibited limited stability during some steps of the elevated temperature (ET) SPPS of the H-1-21-O-Cam-L-NH2 fragment. An SPPS process to obtain H-1-21-HMBA-K fragment in good yield and purity was developed and this peptide was used with the H-22-39-NH2 fragment in a large scale omniligase-1 catalyzed ligation affording 53 g of crude exenatide with excellent yield and high turnover numbers (TON). The crude product was purified to obtain exenatide API, demonstrating that CEPS can be used for large scale manufacturing of therapeutic peptides in economically as well as environmentally sustainable manner. Cost of manufacturing, complete E factor (cEF) and Carbon Intensity (CI) were determined for all process routes herein vs. a conventional and a lab scale CEPS benchmark processes. This revealed that the CEPS exenatide processes which employed the H-1-21-HMBA-K fragment were not only successfully scaled but also improved in terms of economy as well as environmental footprint compared to both benchmark process routes.


 Please wait while we load your content...
Please wait while we load your content...