Electrochemical intramolecular C–H/N–H functionalization for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones†
Abstract
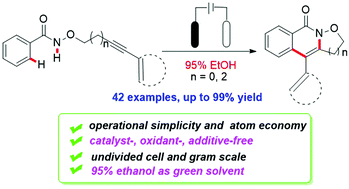
A general and practical protocol for the construction of isoxazolidine-fused isoquinolin-1(2H)-ones has been described by electrochemical-oxidation-induced intramolecular annulation via amidyl radicals. In an undivided cell, isoquinolinones could be easily generated from various available amides bearing CONHOR groups under metal-free, additive-free and external oxidant-free conditions. Moreover, this transformation proceeded smoothly by using cheap 95% ethanol as the green solvent and could be extended to the gram scale.


 Please wait while we load your content...
Please wait while we load your content...