Regio- and stereoselective multi-enzymatic aminohydroxylation of β-methylstyrene using dioxygen, ammonia and formate†
Abstract
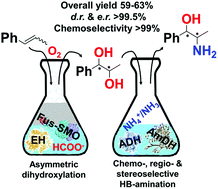
We report an enzymatic route for the formal regio- and stereoselective aminohydroxylation of β-methylstyrene that consumes only dioxygen, ammonia and formate; carbonate is the by-product. The biocascade entails highly selective epoxidation, hydrolysis and hydrogen-borrowing alcohol amination. Thus, β-methylstyrene was converted into 1R,2R and 1S,2R-phenylpropanolamine in 59–63% isolated yields, and up to >99.5 : <0.5 dr and er.


 Please wait while we load your content...
Please wait while we load your content...