Balanced distribution of Brønsted acidic sites and Lewis acidic sites for highly selective conversion of xylose into levulinic acid/ester over Zr-beta catalysts†
Abstract
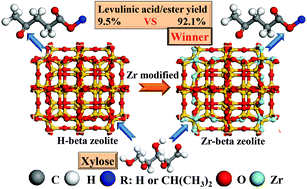
Levulinic acid (LA), a platform chemical, can be produced from acid-catalyzed conversion of C6 sugars such as glucose. Production of LA from C5 sugars such as xylose is also of importance, as this could increase the overall yield of LA from biomass. In this study, selective conversion of xylose into levulinic acid/ester via a one-pot reaction was achieved with Zr-beta as the catalyst and isopropanol as the reaction solvent. The yield of levulinic acid/ester reached 92.1% under optimized conditions. The Brønsted acidic sites in the Zr-beta catalysts catalyzed the dehydration of xylose to furfural, while the Lewis acidic sites as well as basic sites catalyzed transfer hydrogenation of furfural to furfuryl alcohol (FA). The Zr-beta catalyst with the most abundant Lewis acidic sites, the lowest ratio of Brønsted acidic sites to Lewis acidic sites and the suitable amount of basic sites was the most active and selective one for catalyzing conversion of xylose into LA. The conversion of furfural into FA was a rate-determining step, the occurrence of which over the Zr-beta catalyst minimized the chance for the polymerisation of furfural. Water formed from the dehydration of sugars played important roles in enhancing the yields of levulinic acid/ester by affecting the formation/distribution of Brønsted acidic sites. In addition, the low ratio of Brønsted to Lewis acidic sites in the catalyst also alleviated polymerisation reactions. The balanced distribution of the Brønsted/Lewis acidic sites and basic sites is essential for achieving the selective conversion of xylose into levulinic acid/ester.


 Please wait while we load your content...
Please wait while we load your content...